The Coming Era of Cell Therapy, How iPSC Opens Passage to a New World? |JD Capital Insights
2024-02-18

Shared by.
Research Team | JD Capital Life Science Investment Department
Managing Director Zhang Yunfeng
Investment Manager Wang Boyu
Data shows that there are about 8.9 million heart failure patients in China, with nearly half of the patients dying within five years of diagnosis. The five-year survival rate is lower than most malignant tumors.
In the past, heart failure was incurable, medical means could only delay its progress. In recent years, with technology advancements, new treatments have emerged for heart failure patients.
In May 2020, published on Nature, two end-stage heart failure patients in China received reprogrammed stem cell therapy, they recovered and were discharged from hospital a year later. It is reported these patients were injected with cardiomyocytes derived from iPSCs in May 2019 at Nanjing Gulou Hospital. The surgery was completed by Professor Wang Dongjin, the director of cardiothoracic surgery at the hospital. This is known to be the world's first clinical application of iPSC technology for treating damaged hearts."
iPSC, known as induced pluripotent stem cells, are a type of pluripotent stem cells artificially reprogrammed, and de-differentiated from somatic cells. iPSCs have the potential for multi-directional specialization and strong self-replication. When conditions are met, they can differentiate into various functional cells and can be cultured in vitro to obtain millions or even billions of clinically relevant cell phenotypes.
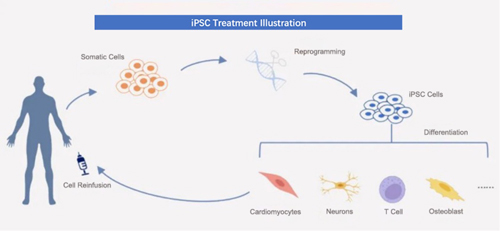
In 2006, Kyoto University professor Shinya Yamanaka, using viral vector, transferred a combination of four transcription factors (Oct4, Sox2, Klf4, and c-Myc) into mice skin cells, and successfully induced iPSC. This won Shinya Yamanaka the Nobel Prize in Physiology or Medicine six years later in 2012.
Subsequently, large-scale research on iPSCs has been comprehensively carried out throughout the globe. Among them, Chinese research teams such as those led by Professor Pei Duanqing; Professor Deng Hongkui; Professor Ding Sheng, and others made significant contributions in the field of chemical small molecule-induced iPSCs.
Currently, iPSCs have been trialed in various clinical treatments for diseases, indications include heart failure, stroke, knee osteoarthritis, Parkinson's disease, leukemia, etc. In April 2022, China approved for the first time a cell therapy product derived from iPSCs to enter clinical trials.
Additionally, iPSCs can also be used by pharmaceutical companies in drug screening CRO (research outsource) services, and by research institutes in establishing disease models for scientific research and more.
JD Capital research believes that iPSC technology, similar to genome editing, belongs to fundamental technologies capable of engineering life, presenting systemic investment opportunities.
Currently, the iPSC industry primarily generates revenue from non-medical-grade sales, targeting pharmaceutical companies and research institutions. With the market launch of cell therapy products, there is enormous potential for market development.
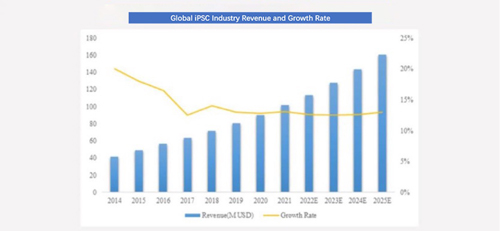
According to incomplete statistics, global revenue of iPSCs in 2019 was about 80 million U.S. dollars, and it is expected to reach 160 million U.S. dollars by 2025. In the next five years, compound annual growth rate of global revenue is expected to be 12.26%
Ⅰ. The Era of Cell Therapy: iPSC may be the best solution.
In 2015, the renowned oncologist and author Siddhartha Mukherjee mentioned in his speech "Forget the Pill, Cell Therapy is Coming" that pharmacotherapy once brought a significant change in human history, curing diseases such as pneumonia, syphilis, and tuberculosis.
The model of pharmacotherapy is - “disease, drug, target”. E.g., penicillin is used to treat pneumonia and kill microbes.
However, the pharmacotherapy model does not always work. Nature offers another perspective on diseases: cells combine to form organs, organs come together to form life, ultimately creating a rich ecosystem.
Mukherjee believes that for many non-infectious diseases, including chronic degenerative diseases like diabetes, high blood pressure, and heart disease, compared to "killing something," what people may need to do is "cultivate something," namely cells. The model of cell therapy is "cells, tissues, environment."
JD Capital views cell therapy as one of the important methods of future disease treatment. With unique mechanisms, the main advantages are: significant and lasting efficacy, personalized treatment, and the ability to treat diseases that traditional pharmacotherapy cannot address.
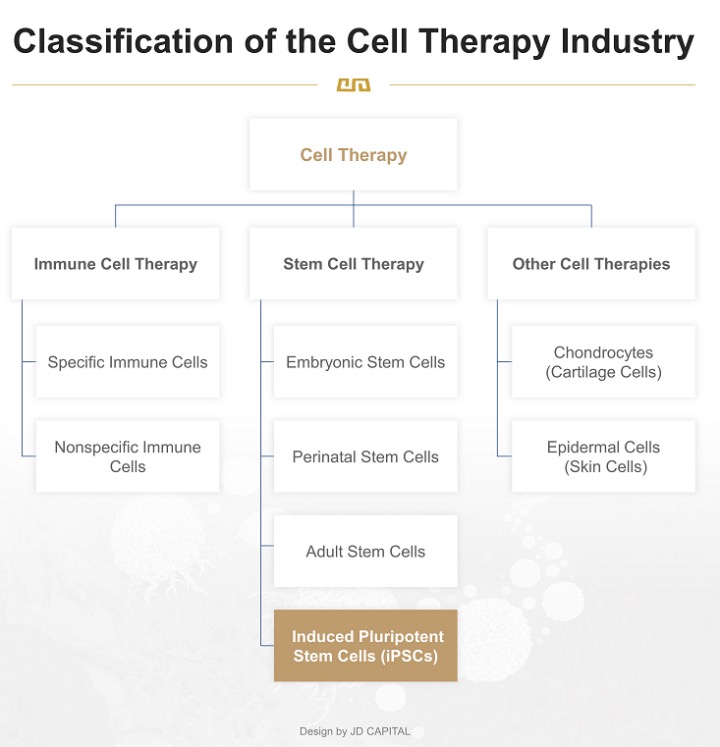
Generally, cell therapy is composed of immune cell therapy, stem cell therapy, and other forms of cell therapy.
In the cell family, stem cells are the constructor and repairers, immune cells are the defenders. They work together to build a healthy human body.
When the human body is still growing, stem cells continuously differentiate and specialize into new cells, increasing the variety and number of cells. Once adulthood is reached and the body stops growing, stem cells can timely replace and renew aging or damaged cells. Moreover, when external intruders (such as bacteria and viruses), or internal rebels (such as normal cells mutating into cancer cells) appear, immune cells rapidly identify and eliminate them.
Regarding stem cells, they can be classified based on their origin into embryonic stem cells, perinatal stem cells, and adult stem cells:
l Embryonic stem cells are derived from embryos.
l Perinatal stem cells are extracted from tissues such as umbilical cord blood, the umbilical cord, and the placenta at the time of birth.
l Adult stem cells are extracted from adult body tissues, including bone marrow, fat, nerves, skin, etc.
In Pei Duanqing’s – researcher at Guangzhou Institutes of Biomedicine and Health, China Academy of Sciences– words, “Adult stem cells are like the trunk of a large tree, while embryonic stem cells are the roots. Embryonic stem cells not only maintain the ability for unlimited self-renewal but can also differentiate into various tissue cell types in the body, making them considered the most clinically valuable 'universal cells'".
However, since embryonic stem cells need to be extracted from embryos, there are ethical risks in its clinical application. iPSCs possess similar differentiation potential to embryonic stem cells and can be induced from adult somatic cells. This resolves the longstanding ethical issues in regenerative medicine and the rejection problems associated with allogeneic transplantation, making iPSCs more accessible and versatile for both generic and personalized therapy, thus having much broader application prospects.
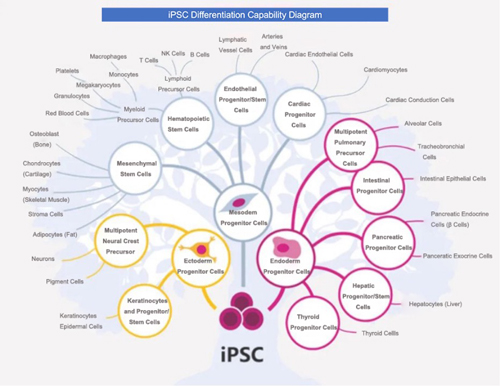
iPSCs can also differentiate into immune cells. Currently, human iPSC-derived CAR (Chimeric Antigen Receptor) -T, CAR-NK, CAR-M, and other cell therapies have been developed and are gradually moving towards clinical application.
A milestone event occurred in October 2020 when a research team from Japan Chiba University and RIKEN announced the successful completion of world's first transplantation of iPSC-derived NKT cells into a cancer patient. This was also Japan's first attempt to use iPSCs for cancer treatment.
Currently, clinical applications of immune cell therapy products are mostly based on autologous immune cells from patients. Autologous immune cell therapy products are highly individualized, and has a lot of limitations, i.e., limited production scale, difficulties in quality control, long processing cycles, and less effective in patients with rapidly progressing conditions. iPSC technology, on the other hand, can produce "ready-made" allogeneic immune cell therapy products to reverse physical injury or disease.
iPSCs can also be used to culture organoids. Previous JD Capital insight - The 'Mini-Organs' in a Petri Dish: Supporting Another Potential Track in the Biomedical Industry? - touched on organoids having practical values in precision medicine, new drug development, infection biology, toxicology research, and even public health safety and military fields.
Furthermore, iPSCs can be combined with CRISPR and other genome editing technologies to accurately and selectively knock out or insert genes in many types of cells, including base editing, correction of faulty genes in stem cells, adding new pathways for personalized medicine.
In JD Capital’s view, iPSCs, as an emerging technology, integrates immune cell therapy, stem cell therapy, organoid culture, and genome editing, demonstrating immense applicable potential. Currently, iPSCs might just be the optimal cell therapy solution.
II. The “mountains” obstructing application: technology and cost
Theoretically, any type of somatic cell can be reprogrammed, but iPSCs are primarily prepared from easily obtainable cells, such as those from skin or blood.
For example, the classic four-factor method of iPSC generation involves four main steps: isolation and cultivation of somatic cells → construction of mediating vectors → introducing reprogramming factors and cell culture → screening and identification of iPSCs.
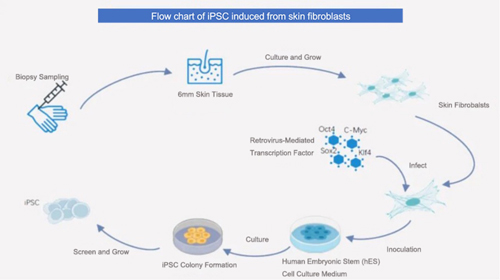
From the initial classic four-factor method to various improvements and alternatives, there are now over 30 different technical approaches to induce iPSCs.
However, the commercial application of iPSCs is still in its early stages. Particularly in terms of safety, Professor Shinya Yamanaka mentioned in his academic papers that tumorigenicity, immunogenicity, and heterogeneity are inherent characteristics of iPSCs and represent three major challenges for their application.
Tumorigenicity: one of iPSCs' significant advantage is its unlimited proliferation potential, making them ideal for preparing various types of human cells used in transplantation. However, this characteristic is a double-edged sword. If the cells continue to proliferate after transplantation, they may cause tumors.
Immunological rejection is an unavoidable problem in any cell therapy. Although the low immunogenicity of autologous iPSCs (induced from a patient's own cells) has been confirmed by research, the majority of current iPSC projects involve allogeneic "off-the-shelf" iPSCs (induced from someone else's cells), which bring greater immunogenicity issues.
Although pluripotent stem cells all have pluripotency and unlimited proliferation potential, pluripotent stem cells from different cell lines vary in morphology, growth curves, gene expression, and tendencies to specialize into various cell lineages. This heterogeneity poses obstacles for downstream applications.
According to interviews conducted by JD Capital, the industry currently has relatively mature technological solutions for the problems of iPSC tumorigenicity and immunogenicity, but the technology to address heterogeneity is not yet mature.
Solutions for tumorigenicity include establishing effective in vitro targeted differentiation methods, maintaining strict purification procedures, and conducting subsequent long-term tumorigenicity experiments for safety assessment. Similar to standard tumor detection methods, iPSC tumorigenicity detection includes tumor markers and imaging.
Currently, The main method to address immune rejection is establishing iPSC super-donor banks and utilizing HLA (human leukocyte antigen) cloaking techniques.
Allogeneic transplantation requires iPSCs reprogrammed from HLA-matched donors, and finding fully compatible matches is time-consuming, difficult, and costly.
There are "super donors" in the population whose homozygotes for 5 loci have high HLA haplotype frequencies. Cells prepared from these rare donors can cover a high number of recipients, to some extent avoiding extensive individual prospective typing, thus reducing the cost and time of iPSC manufacturing.
Several countries and regions have established iPSC super donor banks. In 2018, China prepared its
first pluripotent stem cell line induced by a "super donor." Similar to the United States, the HLA
complexity in China's population means its super donors can only cover about 50% of the Chinese
population, so there is a need to establish personal iPSC banks, for future reserves.
Super donor banks can provide haploidentical matches (half-match), but both fully matched and half-matched transplantation carry risks of GvHD (graft-versus-host Disease) and HvG (host-versus-graft) reactions.
On the other hand, due to the high variability of HLA genotypes in the population, establishing iPSC banks are relatively difficult. CRISPR/Cas9 and other gene editing technologies can edit heterozygous iPSCs to establish HLA homozygous cells, a technique known as HLA cloaking.
However, this technology is not yet mature and is difficult to turn into marketable products. Moreover, altering HLA may affect the therapeutic effect and functions of iPSC cells and cannot eliminate 100% immune rejections.
To overcome heterogeneity, some researchers have attempted to switch pluripotent stem cells from a "primed" state to a "naïve" state. Various methods have been reported for converting iPSCs to a "naïve" or ground state pluripotency, including combinations of growth factors and chemical inhibitors.
However, this technology is also in its infancy, and the converted naïve human stem cells may proliferate too rapidly, and in rare cases, chromosomal abnormalities may occur.
This technology can also lead to the loss of imprinting. A characteristic of naïve human stem cells is overall low methylation, which will be remethylated when re-differentiated to a primed state. However, most imprinted genes are erased in re-primed cells, and this abnormal imprinting may hinder their clinical application.
For autologous iPSCs, there are significant challenges in terms of time and economic cost.
JD Capital observes, iPSC usually takes two months to establish a cell line, followed by three months to grow it. Then, another three months differentiating the iPSCs into specific target cells, which are further grown over several months. Finally, another 6 months for these cells to undergo testing that ensure they do not form tumors. Moreover, all of these steps must be conducted under GMP (Good Manufacturing Practice) conditions, significantly increasing the cost.
In addition, while there are many technical routes for inducing iPSCs, the industry is still in the exploratory stage regarding the production techniques, reprocessing methods, and transplantation methods for iPSCs.
JD Capital's research found, for iPSC production a major challenge lies in achieving high purity in cell purification. For reprocessing methods and transplantation methods, an area that needs further exploration is concerned with PSC-derived cells not able to directly go into the human body for treatment. They instead need to be introduced in the form of microcapsules or cell clusters. It needs to be determined, based on transplantation site, the most beneficial reprocessing method.
III. “fundamental infrastructure “underlying the passage to application.
Apart from clinical applications, iPSCs have two main applicable fields: to pharmaceutical companies providing drug screening CRO services, and to research institution builidng disease models.
1,Drug Screening CRO Services:
Traditional drug screening primarily relies on standard cell models or animal models. However, these methods have limitations such as mismatch with pathology, high costs, and long cycles. iPSCs, which have the advantages of unlimited proliferation, specific differentiation, and accurate recapitulation of pathological state, can enhance efficiency, and reduce costs in drug screening.
In more detail, during early stage of drug development, the high attrition rate is often related to inaccurate results from suboptimal in vivo screening tests. Currently, a barrier to improving R&D efficiency is related to using non-human animal models to evaluate target organ toxicity, which cannot accurately apply to prediction and assessment of human toxicity.
For example, rodent experiments are often used to predict human cardiotoxicity. However, these experiments are limited by high costs, low throughput, and limited relevance to humans, and their ion channels differ from humans. In Vitro experiments with animal myocardial cells also do not adequately explain results observed in human experiments.
iPSCs offer the possibility to overcome these obstacles. They exhibit many characteristics similar to normal in vivo cells, such as morphological structure, gene expression, functional ion channels, receptor expression, and electrophysiological properties. These characteristics support the use of iPSC-differentiated myocardial cells for drug-induced cardiotoxicity screening.
More importantly, large-scale drug screening typically requires a large number of cells. Since iPSCs can proliferate indefinitely in vitro, iPSCs disease models and differentiated functional cells can be provided unlimitedly. Additionally, in standardized processing, consistency and stability can be maintained between batches of cells.
2,Building Disease Models:
iPSC technology can reprogram patients' somatic cells into patient-specific iPSCs, which carry specific disease-related and genetic information. These can then be further induced to differentiate into disease-related cell types or organoid models, thus constructing disease models in a dish.
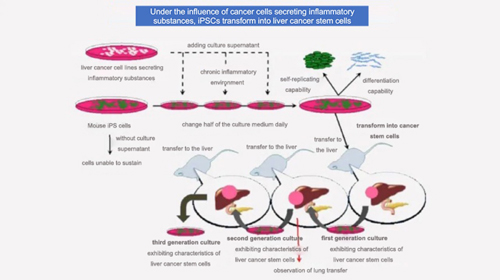
For example, a research team from Okayama University Graduate School in Japan used human liver cancer cell line secreting various inflammatory substances culture supernatant, to culture mouse iPSCs. The iPSCs were then induced into tumor stem cells, which were transplanted into the livers of nude mice to successfully created liver cancer model. This was the first successful creation of a liver cancer model in the world.
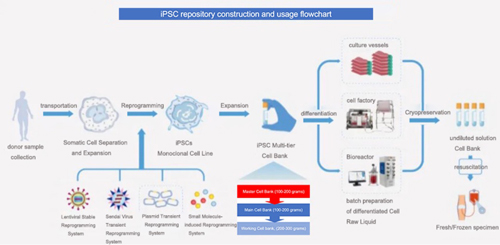
Regardless of how iPSCs are applied, there is a preliminary task: establishing a cell bank.
Cell bank/repository building typically refers to gradually increasing the number of containers or expansion to container volume, so cells can be grown to generate sufficient quantity, which are then distributed into an adequate number of containers.
Cell banks include the primary cell bank (PCB, also known as cell seed), master cell bank (MCB), and working cell bank (WCB). Manufacturers can prepare their own cell banks or obtain them externally.
The establishment process of an iPSC bank typically includes: sample collection, isolation and proliferation of somatic cells, reprogramming induction, screening of iPSC monoclonal cell strains, proliferation, and testing etc.
The sources for adult iPSCs cells mainly include various adult stem cell banks, medical institution banks, and various human biorepositories.
Currently, the global establishment of iPSC banks is in its initial stage, primarily focused on public bank (i.e., super donor banks) construction. Private banks have significant development potential in the future.
JD capital observes, only seven provinces/cities in China, that of Shandong, Beijing, Shanghai, Tianjin, Guangdong, Zhejiang, and Sichuan, have legal umbilical cord blood banks, and no new licenses are being issued. These cord blood banks are backed by private enterprises, and those with licenses have advantages in conducting iPSC private repository business.
In the short term, iPSC private repository may be targeting the high-end consumer group.
JD Capital estimates that excluding rent, utilities, and laboratory personnel salaries, the production cost of a single iPSC strain, which includes cell culture reagents, laboratory consumables (such as centrifuge tubes, culture dishes), cell cryopreservation consumables (such as special cryopreservation fluid, cryopreservation tubes), and iPSC identity testing (such as pluripotency, karyotype tests), is between CNY 3,000 to 5,000 yuan. This means that the unit price of commercial products for private repository will be over CNY ten thousand yuan.
IV. Under global competitive landscape, challenges, and opportunities for Chinese companies
According to JD capital research, there are mainly two commercial models for iPSC: one is using iPSC for cell therapy and advancing clinical pipelines, and the other involves technical services such as iPSC induction, establishment, proliferation, differentiation, and banking/repository (including customized R&D and production, i.e., CDMO), as well as the sale of cell strains and cell culture media.
For iPSC therapy companies, competitive factors are concentrated in the following dimensions.
1, Mature and stable cell production and quality control processes.
To ensure the safety of iPSC therapeutical products, it is essential to start by controlling the quality of raw materials into cell culture and optimizing the cultivation, differentiation, and batch production processes of iPSCs. Additionally, the processing of stem cells must be carried out in a completely sterile environment, which imposes high standards for laboratory and production facilities. According to national regulations, the production of cell therapy products must be conducted in laboratories or production workshops with a cleanliness level of B+A.
2, Quality review by the National Institutes for Food and Drug Control (NIFDC) in China.
The National Institutes for Food and Drug Control (NIFDC) in China is the state-mandated institution for the quality inspection of biopharmaceuticals. It is also the only clinical-grade cell inspection organization recognized by 3A-Grade hospitals. If a company receives cell quality endorsement from the NIFDC, it indicates that its stem cell quality has reached the clinical level.
3, Selection of indications.
Companies in the iPSC sector have developed pipelines covering multiple indications. Of which, heart failure, stroke, and Parkinson's disease have no cure, and tumors for which there are no effective treatments, making these indications more valuable in the market. By contrast, Type 1 Diabetes and leukemia, alternative therapies are already available, and osteoarthritis of the knee does not severely affect quality of life, the willingness of patients to pay is thus relatively lower.
4, Hospital cooperation and endorsement
Most stem cell clinical research projects are conducted in collaboration between stem cell companies and hospitals. Whether or not a company partners with well-known hospitals is an important basis for assessing the strength of an iPSC company.
5, Pipeline progress.
Currently, around the globe, no iPSC therapy has been approved for commercialization. There are 19 products that have either started clinical trials or obtained implied permission for clinical trials (IND). The fastest progressing pipeline is the Australian Cynata's CYP-004, which uses iPSC-derived mesenchymal stem cells (MSC) for the treatment of osteoarthritis and has entered phase 3 clinical trials.
6, Patents
Japan's FCDI (Fuji Cellular Dynamics) has a large number of patents in the processing, proliferation, and induced differentiation of iPSCs, especially in the patents of CAR-PSC differentiation into T cells or NK cells, which currently have a broad scope of rights claims. Other companies need to be cautious to avoid infringement.
Today, looking at the global competitive landscape, China, the United States, and Japan are leading in the iPSC field, with a relatively small gap between China and the US. In terms of the number of companies with approved INDs, there are 9 in the US and 6 in China.
As star companies in the global iPSC industry, the development of Fate and Century in the United States is currently unsatisfactory.
Fate, established in 2007 and listed on NASDAQ in 2013, is a pioneer in the iPSC field and has created the world's first iPSC-derived universal CAR-T therapy.
In April 2020, Fate reached a collaboration with Johnson & Johnson: Janssen, a subsidiary of J&J, contributed proprietary antigen-binding domains for four cancer-related antigen targets, while Fate researched and developed iPSC-derived CAR-NK and CAR-T cell candidate products. After the candidate drugs is submitted INDs, Janssen would exercise its exclusive commercialization rights to the relevant products. If the collaboration continues successfully, Fate would receive up to $3.1 billion in financial support from Janssen.
However, fortune did not favor Fate. In January 2023, Fate announced that it rejected J&J's renewal terms and terminated the collaboration agreement, cutting about 60% of its workforce and shutting down most of its research projects to focus only on key operations.
Media reports indicate that Fate's clinical phase one trials have been stalled for many years. The reason its iPSC products not progressing to phase two or three clinical trials (critical clinical tests) is that they did not meet the safety and quality control requirements of the FDA (U.S. Food and Drug Administration). The slower-than-expected clinical progress may have been the catalyst for Fate's breakup with J&J.
On the other hand, Century, established in 2018 and listed in 2021 with iPSC as its stock abbreviation, to date not a single product has entered the clinical stage, following a similar path to Fate: its stock price dropped by over 90%, and it needs to significantly cut staff and pipeline to survive.
JD Capital believes, for any emerging technology, there needs to be entrepreneurs to push for progress and undertake risks. The essence to the current development for iPSC therapy companies is to overcome technical difficulties and solve safety and efficacy issues of products. If certain technologies cannot make breakthroughs, it's necessary to cut losses in a timely manner, concentrate efforts and resources on a few key pipelines with higher chances of success, and quickly push them towards commercialization to form a virtuous cycle.
In the journey towards a new world, compared to the challenges of long R&D cycles and uncertain market entry faced by iPSC innovative drugs, JD Capital believes that iPSC banks/repository construction and other technical services have a "infrastructure" property (the building blocks), and in the current stage, may be worthy of more investment attention.
Of-which, bio-repository construction business is large in scale, low in commercialization difficulty, and also a prerequisite for the popularization of "universal" cell therapies.
